 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Phenylethyl acetate | |
| Other names
Phenethyl acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.830 |
| EC Number |
|
| KEGG | |
| MeSH | C054590 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Density | 1.088 g/cm3 |
| Melting point | −31.1 °C (−24.0 °F; 242.1 K) |
| Boiling point | 232.6 °C (450.7 °F; 505.8 K) |
| Hazards | |
| GHS labelling: | |
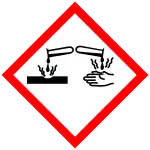  | |
| Danger | |
| H318, H319 | |
| P264, P280, P305+P351+P338, P310, P337+P313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phenethyl acetate is the ester resulting from the condensation of acetic acid and phenethyl alcohol. Like many esters, it is found in a range of fruits and biological products.[1] It is a colorless liquid with a rose and honey scent and a raspberry-like taste.[2][3]
References
- ↑ "Phenethyl acetate". Sigma-Aldrich. Retrieved 11 February 2016.
- ↑ Burdock, George A. (1996). Encyclopedia of food and color additives. Boca Raton [u.a.]: CRC Press. p. 2152. ISBN 9780849394140. Retrieved 23 March 2016.
- ↑ Surburg, Horst; Panten, Johannes (2016). Common Fragrance and Flavor Materials: Preparation, Properties and Uses (6 ed.). John Wiley & Sons. ISBN 9783527693184. Retrieved 23 March 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.